EVRENZO safety profile evaluated in over 6,800 patients* with NDD or DD1
-
The safety profile of EVRENZO has been evaluated in an extensive series of clinical studies1
NDD patients Hb correction ALPS
(N=594)ANDES
(N=916)OLYMPUS
(N=2,760)DOLOMITES
(N=616)tDD patients ESA conversion Hb correction ESA conversion and Hb correction PYRENEES
(N=834)SIERRAS
(N=740)HIMALAYAS
(N=1,039)ROCKIES
(N=2.101)Placebo controlledESA controlledDD, dialysis-dependent; ESA, erythropoiesis-stimulating agent; NDD, non-dialysis-dependent.
*The safety population analysed included all randomised patients who received ≥1 dose of study drug.Reference
- EVRENZO SmPC.
- EVRENZO SmPC.
-
Cardiovascular safety profile has been investigated in both non-dialysis-dependent and dialysis-dependent CKD1
The cardiovascular safety of EVRENZO was assessed in a meta-analysis of adjudicated major adverse cardiovascular events:1
MACE: a composite of all-cause mortality (ACM), non-fatal myocardial infarction (MI) and/or stroke
MACE+: a composite of ACM, non-fatal MI, stroke, and hospitalisation for either unstable angina and/or congestive heart failure
ACM: all-cause mortality
Patients with non-dialysis-dependent CKD
Patients with dialysis-dependent CKD
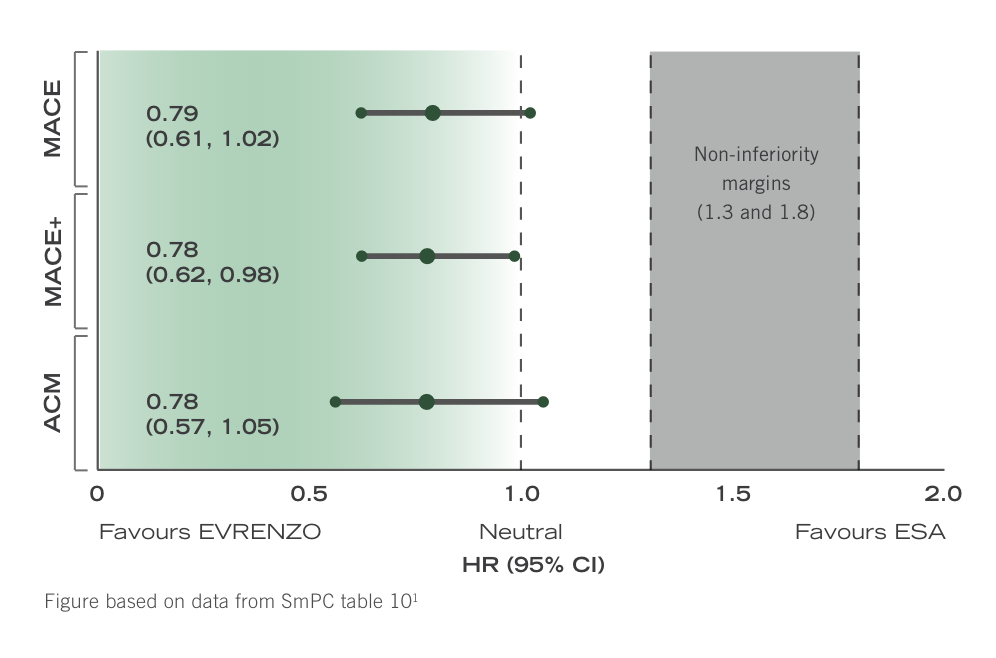
In patients with NDD CKD or patients with IDD CKD (dialysis <4 months) no difference in CV and mortality risk was observed between ESA and EVRENZO arms in patients who were either ESA treated or ESA untreated at baseline.1
Hazard ratios for all tested composite endpoints were between 0.78 and 0.79.
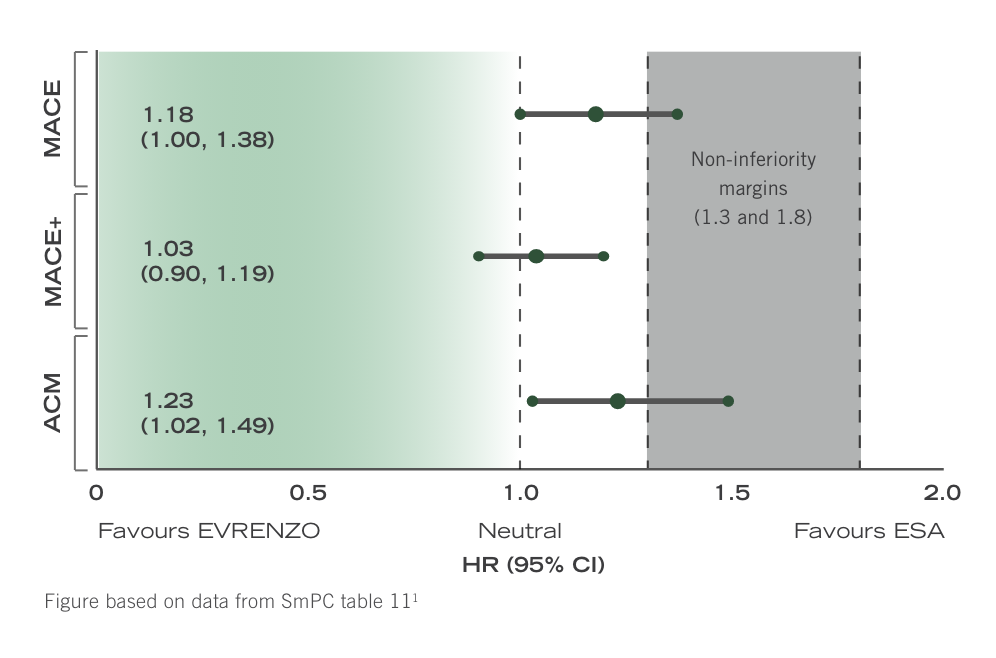
In patients stable on dialysis (>4 months) and stable on ESA, converting to EVRENZO was associated with HRs of between 1.03 and 1.23, favouring ESA.1
A comparison of treatment effects cannot be established in this setting because of the inherent risk in switching. However, it is recommended that patients stable on dialysis and stable on ESA are not switched unless there is a valid clinical reason.1
For more details on the respective hazard ratios and 95% confidence intervals, please see below
ACM, all-cause mortality; CKD, chronic kidney disease; CV, cardiovascular; ESA, erythropoiesis-stimulating agent; HR, hazard ratio; IDD, incident dialysis-dependent; MI, myocardial infarction; NDD, non-dialysis-dependent.
Reference
- EVRENZO SmPC.
- EVRENZO SmPC.
-
No increase in CV or mortality risk with EVRENZO compared with ESA in patients with NDD- and IDD-CKD1
Hazard ratios in ESA-controlled Hb correction pool1
CV events in ESA-controlled Hb correction pool1
OT MACE MACE+ ACM EVRENZO
n=1,083ESA
n=1,059EVRENZO
n=1,083ESA
n=1,059EVRENZO
n=1,083ESA
n=1,059Number of patients with
events, n (%)105 (9.7) 136 (12.8) 134 (12.4) 171 (16.1) 74 (6.8) 99 (9.3) FAIR (/100 PY) 6.5 8.2 8.3 10.3 4.6 6.0 FAIR = follow-up adjusted incidence rate; MACE = death, MI and/or stroke; MACE+ = death, MI and/or stroke with hospitalisations for either unstable angina and/or CHF; PY = patient years.
Data for patients converting from ESA in stable dialysis should be interpreted with caution due to an inherent risk in switching1
Hazard ratios in ESA-controlled ESA conversion stable DD pool1
CV events in ESA-controlled ESA conversion stable DD pool1
OT MACE MACE+ ACM EVRENZO
n=1,594ESA
n=1,594EVRENZO
n=1,594ESA
n=1,594EVRENZO
n=1,594ESA
n=1,594Number of patients with
events, n (%)297 (18.6) 301 (18.9) 357 (22.4) 403 (25.3) 212 (13.3) 207 (13.0) FAIR (/100 PY) 10.4 9.2 12.5 12.3 7.4 6.3 FAIR = follow-up adjusted incidence rate; PY = patient years.
ACM, all-cause mortality; CHF, congestive heart failure; CI, confidence interval; CKD, chronic kidney disease; CV, cardiovascular; DD, dialysis-dependent; ESA, erythropoiesis-stimulating agent; Hb, haemoglobin; HR, hazard ratio; IDD, incident dialysis-dependent; MACE, major adverse cardiovascular event; MACE+, major adverse cardiovascular event including hospitalisations for either stable angina and/or congestive heart failure; MI, myocardial infarction; NDD, non-dialysis-dependent; OT, on-treatment.
Reference
- EVRENZO SmPC.
- EVRENZO SmPC.
-
EVRENZO Summary of safety profile
See EVRENZO Summary of Product Characteristics for full information.
EVRENZO is contraindicated in:1- Hypersensitivity to the active substance, peanut, soya or to any of the excipients
- Third trimester of pregnancy
- Breastfeeding
Not recommended:1
- EVRENZO is not recommended for use in patients with severe hepatic impairment (Child-Pugh class C)
- Lack of effect: In the absence of an addressable cause for an inadequate response to therapy, EVRENZO should not be continued beyond 24 weeks of therapy
- It is not recommended to combine administration of EVRENZO and ESAs as the combination has not been studied
EVRENZO Special Warnings & Precautions1
Cardiovascular and mortality risk
Overall, the cardiovascular and mortality risk for treatment with EVRENZO has been estimated to be comparable to the cardiovascular and mortality risk for ESA therapy based on data from direct comparison of both therapies. Since for patients with anaemia associated with CKD and not on dialysis, this risk could not be estimated with sufficient confidence versus placebo, a decision to treat these patients with EVRENZO should be based on similar considerations that would be applied before treating with an ESA. Further, several contributing factors have been identified that may impose this risk, including treatment non-responsiveness, and, converting stable ESA treated dialysis patients. In the case of non-responsiveness, treatment with EVRENZO should not be continued beyond 24 weeks after the start of treatment. Conversion of dialysis patients otherwise stable on ESA treatment is only to be considered when there is a valid clinical reason. For stable ESA treated patients with anaemia associated with CKD and not on dialysis, this risk could not be estimated as these patients have not been studied. A decision to treat these patients with EVRENZO should be based on a benefit risk consideration for the individual patient.Thrombotic vascular events
The reported risk of thrombotic vascular events (TVEs) should be carefully weighed against the benefits to be derived from treatment with EVRENZO particularly in patients with pre-existing risk factors for TVE, including obesity and prior history of TVEs (e.g., deep vein thrombosis [DVT] and pulmonary embolism [PE]). Deep vein thrombosis was reported as common and pulmonary embolism as uncommon amongst the patients in clinical studies. The majority of DVT and PE events were serious.Cases of cerebrovascular accidents, including fatal cases of cerebral infarction, have been reported in patients treated with roxadustat.
Vascular access thrombosis (VAT) was reported as very common amongst the CKD patients on dialysis in clinical studies.
In CKD patients on dialysis, rates of VAT in EVRENZO-treated patients were highest in the first 12 weeks following initiation of treatment, at Hb values more than 12 g/dL and in the setting of Hb rise of more than 2 g/dL over 4 weeks. It is recommended to monitor Hb levels and adjust the dose using the dose adjustment rules (see SmPC) to avoid Hb levels of more than 12 g/dL and Hb rise of more than 2 g/dL over 4 weeks.
Patients with signs and symptoms of TVEs should be promptly evaluated and treated according to standard of care. The decision to interrupt or discontinue treatment should be based on a benefit-risk consideration for the individual patient.
Seizures
Seizures were reported as common amongst the patients in clinical studies receiving EVRENZO. EVRENZO should be used with caution in patients with a history of seizures (convulsions or fits), epilepsy or medical conditions associated with a predisposition to seizure activity such as central nervous system (CNS) infections. The decision to interrupt or discontinue treatment should be based on a benefit-risk consideration of the individual patient.Serious infections
The most commonly reported serious infections were pneumonia and urinary tract infections. Patients with signs and symptoms of an infection should be promptly evaluated and treated according to standard of care.Sepsis
Sepsis was one of the most commonly reported serious infections and included fatal events. Patients with signs and symptoms of sepsis (e.g., an infection that spreads throughout the body with low blood pressure and the potential for organ failure) should be promptly evaluated and treated according to standard of care.Secondary hypothyroidism
Cases of secondary hypothyroidism have been reported with the use of EVRENZO. These reactions were reversible upon EVRENZO withdrawal. Monitoring of thyroid function is recommended as clinically indicated.Inadequate response to therapy
Inadequate response to therapy with EVRENZO should prompt a search for causative factors. Nutrient deficiencies should be corrected. Intercurrent infections, occult blood loss, haemolysis, severe aluminium toxicity, underlying haematologic diseases or bone marrow fibrosis may also compromise the erythropoietic response. A reticulocyte count should be considered as part of the evaluation. If typical causes of non-response are excluded, and the patient has reticulocytopenia, an examination of the bone marrow should be considered. In the absence of an addressable cause for an inadequate response to therapy, EVRENZO should not be continued beyond 24 weeks of therapy.Hepatic impairment
Caution is warranted when EVRENZO is administered to patients with moderate hepatic impairment (Child-Pugh class B). EVRENZO is not recommended for use in patients with severe hepatic impairment (Child-Pugh class C).Pregnancy and contraception
EVRENZO should not be initiated in women planning on becoming pregnant, during pregnancy or when anaemia associated with CKD is diagnosed during pregnancy. In such cases, alternative therapy should be started, if appropriate. If pregnancy occurs while EVRENZO is being administered, treatment should be discontinued and alternative treatment started, if appropriate. Women of childbearing potential must use highly effective contraception during treatment and for at least one week after the last dose of EVRENZO.Misuse
Misuse may lead to an excessive increase in packed cell volume. This may be associated with life-threatening complications of the cardiovascular system.Excipients
EVRENZO contains lactose. Patients with rare hereditary problems of galactose intolerance, total lactase deficiency or glucose-galactose malabsorption should not take this medicinal product.
EVRENZO contains Allura Red AC aluminium lake which may cause allergic reactions.
EVRENZO contains traces of soya lecithin. Patients who are allergic to peanut or soya, should not use this medicinal product.EVRENZO safety profile in clinical trials
The safety profile of EVRENZO was evaluated in 3,542 non-dialysis dependent (NDD) and 3,353 dialysis dependent (DD) patients with anaemia and CKD who have received at least one dose of EVRENZO in clinical studies. In addition, post-marketing experience data is also comprised.1
Most frequent (≥10%) adverse reactions:1
- Hypertension (13.9%)
- Vascular access thrombosis (12.8%)
- Diarrhoea (11.8%)
- Peripheral oedema (11.7%)
- Hyperkalaemia (10.9%)
- Nausea (10.2%)
Most frequent (≥1%) serious adverse reactions:1
- Sepsis (3.4%)
- Hyperkalaemia (2.5%)
- Hypertension (1.4%)
- Deep vein thrombosis (1.2%)
Adverse reactions observed during clinical studies and/or in post-marketing experience1
System Organ Class (MedDRA) Frequency category* Adverse reaction Infections and infestations Common Sepsis Endocrine disorders Not known Secondary hypothyroidism Metabolism and nutrition disorders Very Common Hyperkalaemia Psychiatric disorders Common Insomnia Nervous system disorders Common Seizures, headache Vascular disorders Very common Hypertension, vascular access thrombosis (VAT)** Common Deep vein thrombosis (DVT) Gastrointestinal disorders Very common Nausea, diarrhoea Common Constipation, vomiting Skin and subcutaneous tissue disorders Not known Dermatitis Exfoliative Generalised (DEG) Hepatobiliary disorders
Uncommon
Hyperbilirubinaemia Respiratory, thoracic, mediastinal disorders Uncommon Pulmonary embolism General disorders and administration site conditions Very common Peripheral oedema Investigations Not known Blood thyroid stimulating hormone (TSH) decreased, Blood copper increased *Very common (≥1/10); common (≥1/100 to <1/10); uncommon (≥1/1,000 to <1/100); rare (≥1/10,000 to <1/1,000); very rare (<1/10,000); not known (cannot be estimated from the available data).
**This adverse reaction was associated with CKD patients who were on dialysis while receiving EVRENZO.MedDRA, medical dictionary for regulatory activities; SmPC, Summary of Product Characteristics.
Reference- EVRENZO SmPC.
-
Initial Hb monitoring similar to other treatments for anaemia of CKD
Thrombotic complications are a known adverse event when increasing Hb levels with treatment for anaemia of CKD.1
- Rapid rises in Hb e.g. >2 g/dL (20 g/L) over 4 weeks, or very high Hb levels (>12 g/dL) can be associated with an increased risk of thrombotic compIications2,3
Recommendations for EVRENZO: Monitor Hb regularly and avoid steep rises
- For patients started on EVRENZO, Hb levels should be monitored every 2 weeks until the target Hb level of 10-12 g/dL (100-120 g/L) is achieved and stabilised, and every 4 weeks thereafter or as clinically indicated.4
- When monitoring Hb levels, the dose of EVRENZO should be adjusted using the dose adjustment rules to avoid Hb level rises of >2 g/dL (>20 g/L) over 4 weeks.4
With EVRENZO, the dose can be individualised to achieve and maintain target levels of 10-12 g/dL (100-120 g/L).4
CKD, chronic kidney disease; Hb, haemoglobin.
References
- Locatelli F et al. Am J Nephrol. 2017;45:187-199.
- Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int Suppl. 2013;3:1-150.
- Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int Suppl. 2012;2:279-335.
- EVRENZO SmPC.
- Rapid rises in Hb e.g. >2 g/dL (20 g/L) over 4 weeks, or very high Hb levels (>12 g/dL) can be associated with an increased risk of thrombotic compIications2,3
