EVRENZO inhibits HIF-PH, thus preventing breakdown of HIF-α and activating the HIF pathway2,3
This induces transcription and translation of genes involved in erythropoiesis2,3
This site is intended only for Healthcare Professionals, particularly those who are experienced in the management of anaemia associated with chronic kidney disease.
The website you have tried to access contains clinical information designed specifically for medical professionals experienced in treating this condition. If you have any questions about your health or treatment, please consult your doctor.

The HIF pathway is the physiological mechanism by which the body responds to low oxygen conditions, such as those experienced at high altitude.
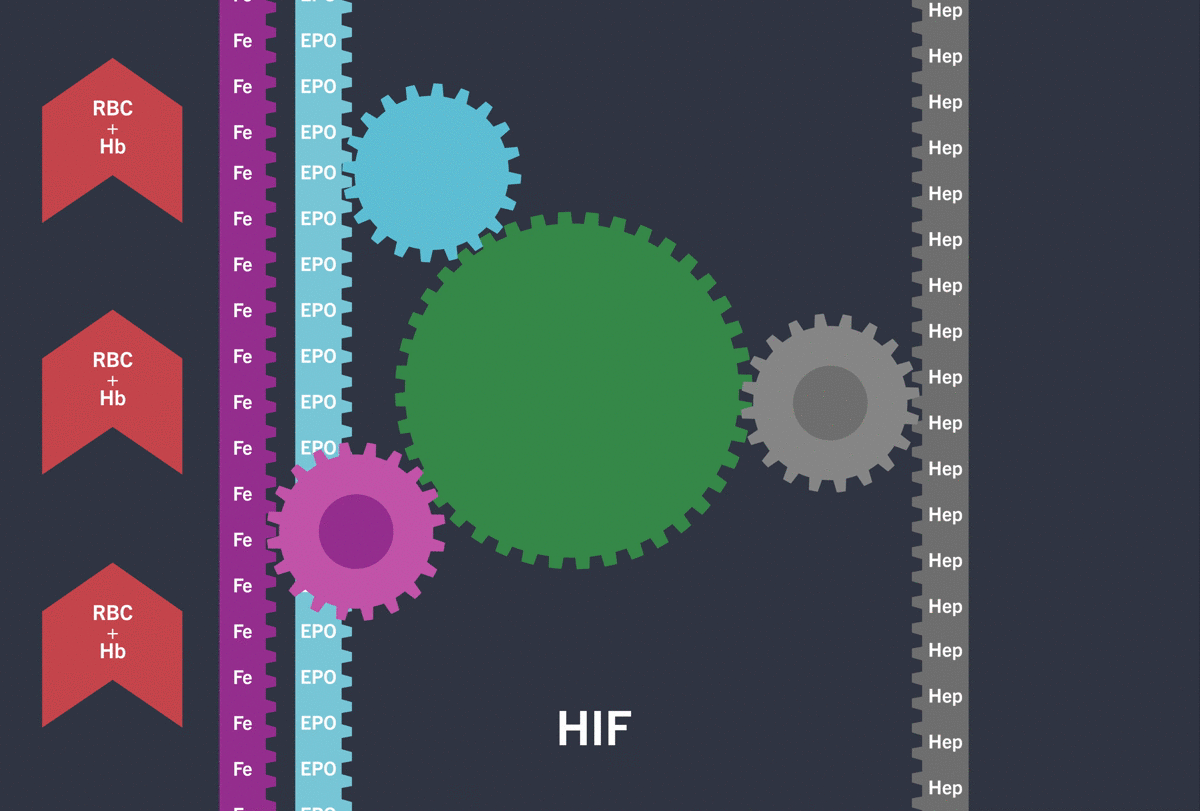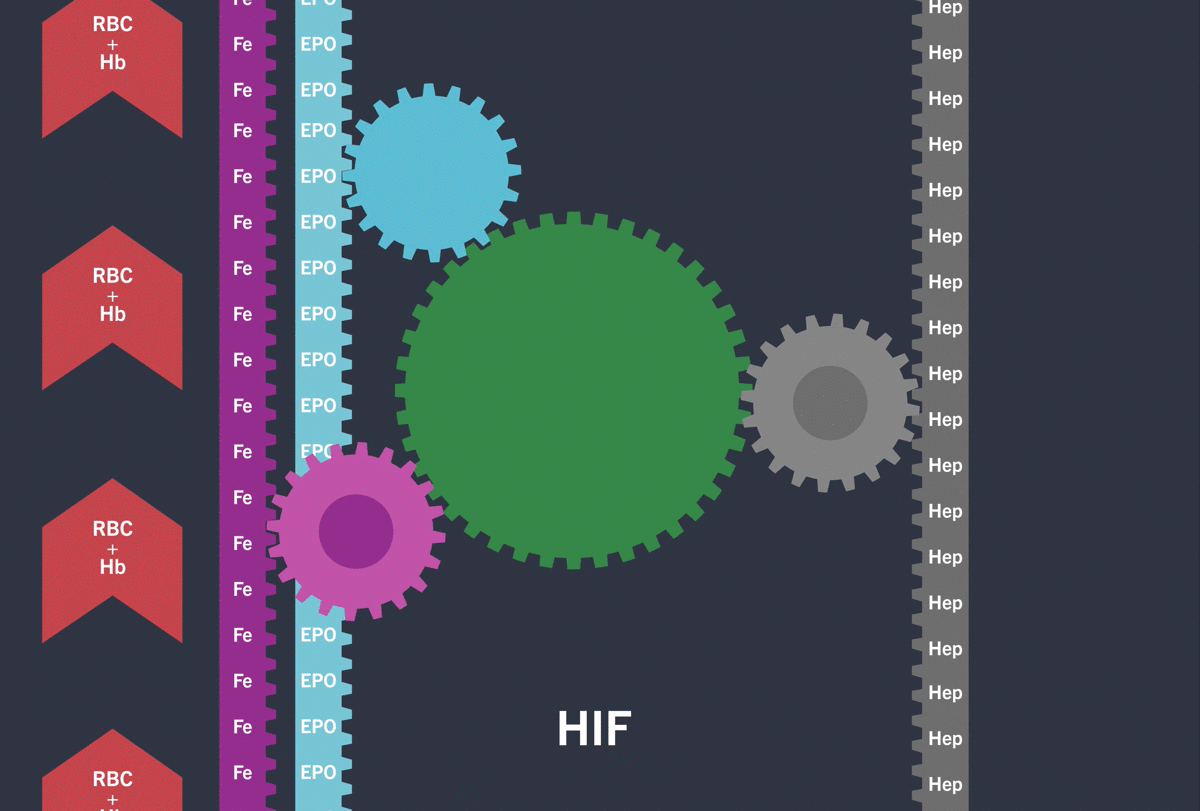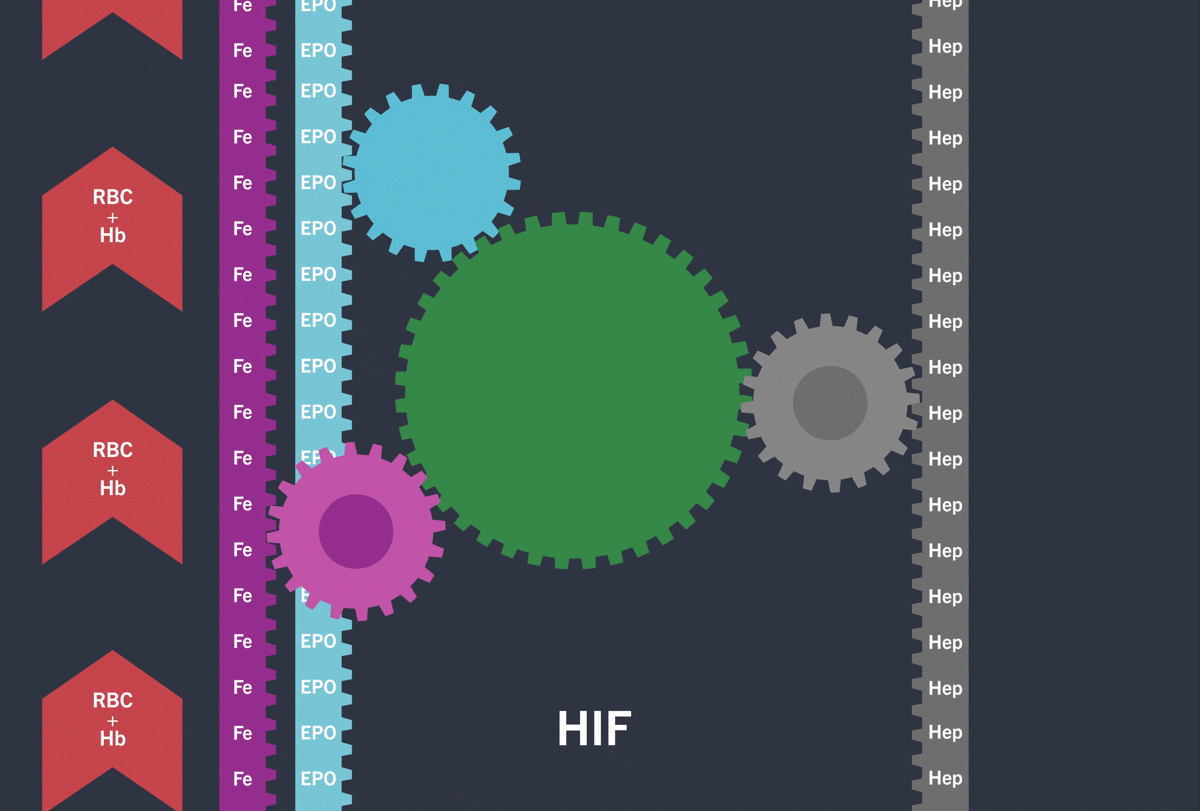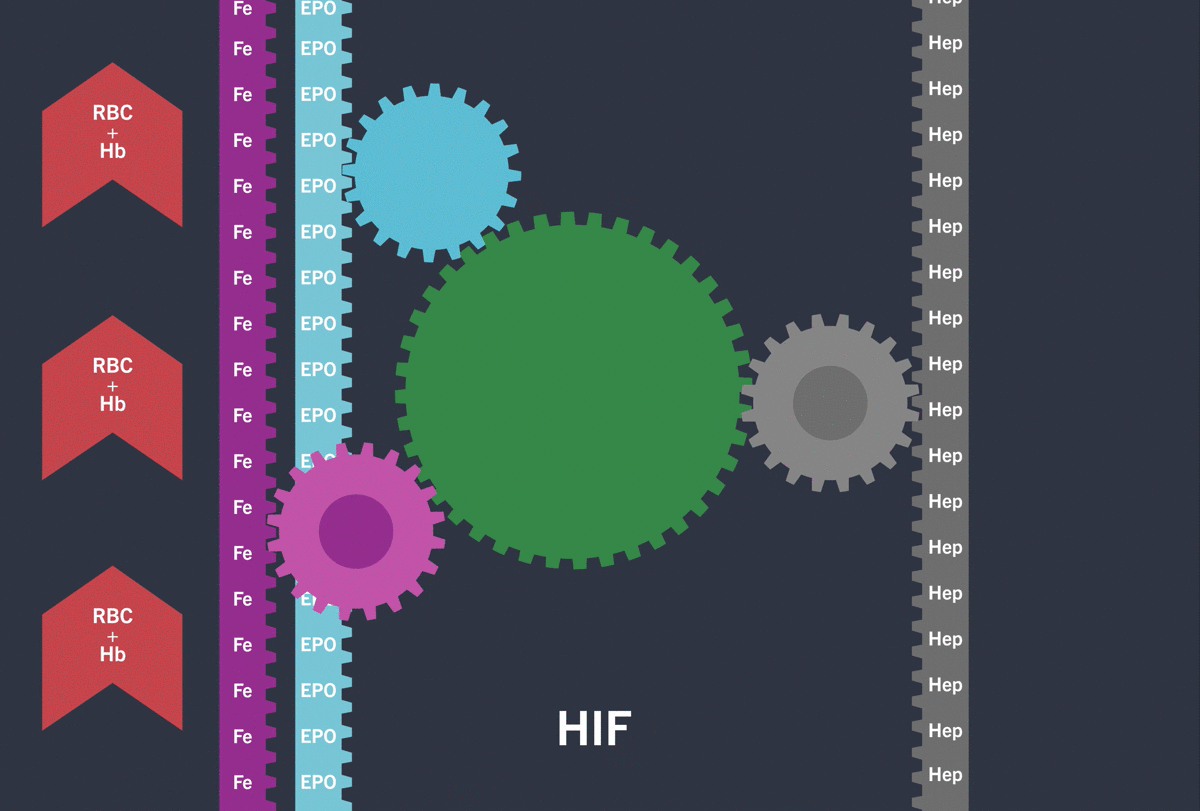
Activation of the HIF-pathway leads to transcription of genes involved in iron (Fe) turnover and the production of Erythropoietin (EPO) resulting in increased red blood cell count (RBC) / haemoglobin (Hb)
Under normoxic conditions, the HIF pathway is not activated: the HIF-α subunit is rapidly degraded via HIF prolyl-hydroxylase (HIF-PH) in the presence of oxygen:3
Normoxic conditions
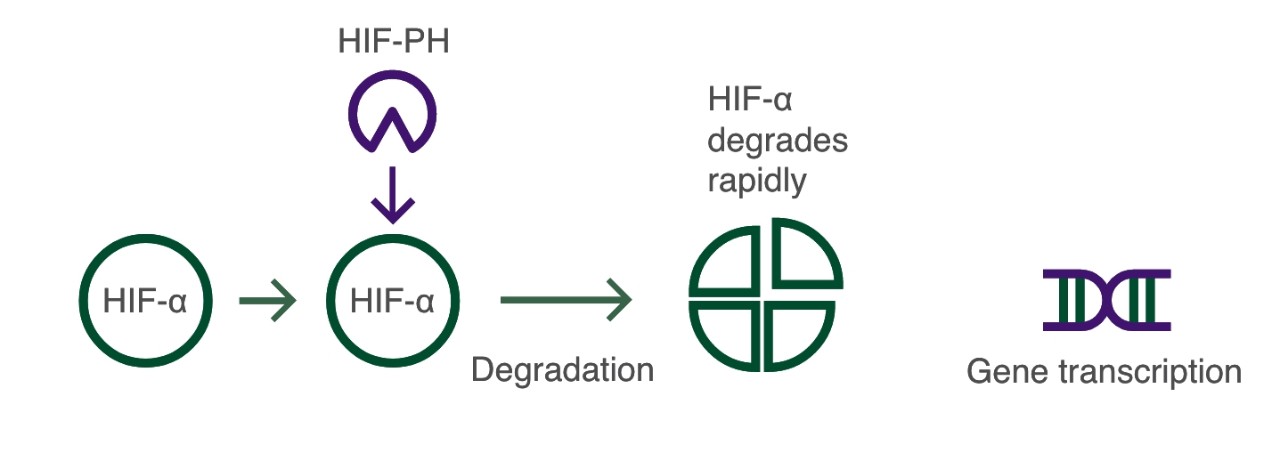
Under hypoxic conditions, the HIF pathway is activated: the HIF-α subunit is not degraded and dimerises with the HIF-β subunit inducing the transcription and translation of target hypoxia response genes involved in erythropoiesis, including:3-5

In CKD, oxygen sensing via HIF is disrupted, and this contributes to the development of anaemia.6
A unique strategy to mimic the physiological response to hypoxia is to inhibit HIF-PH, facilitating the accumulation of HIF-α and the resulting induction of hypoxia response genes.1
CKD, chronic kidney disease; HIF, hypoxia-inducible factor; HIF-PH, hypoxia-inducible factor prolyl-hydroxylase; EPO, erythropoietin.
References
EVRENZO is a HIF-PH inhibitor, a class of drug with an innovative mechanism of action.2
Mimicking hypoxia
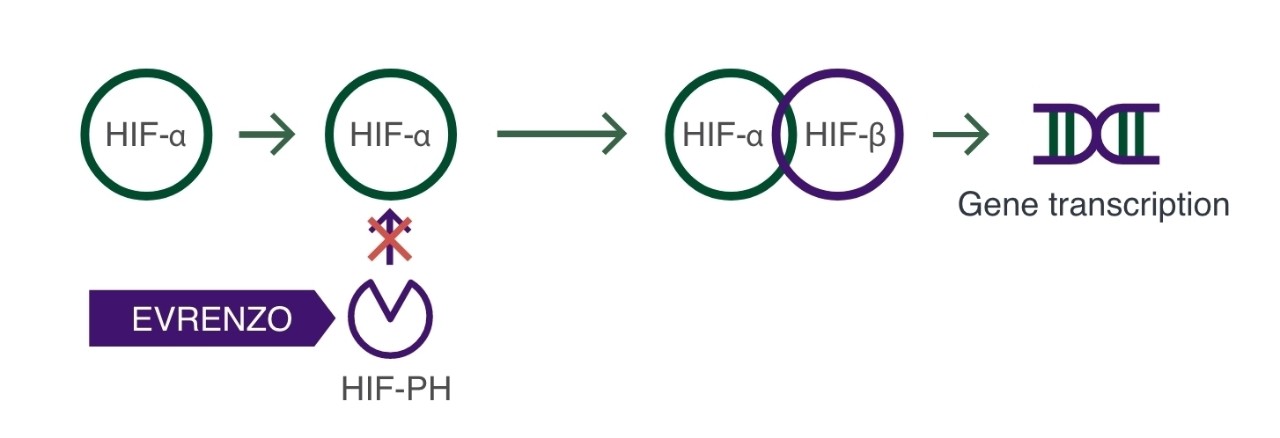
EVRENZO inhibits HIF-PH, thus preventing breakdown of HIF-α and activating the HIF pathway2,3
This induces transcription and translation of genes involved in erythropoiesis2,3
HIF-PH inhibitors induce activation of the genes responsible for erythropoiesis, mimicking a hypoxic state.3
Through the inhibition of HIF-PH, EVRENZO stimulates a coordinated erythropoietic response that not only increases erythropoietin (EPO) levels, but also increases iron mobilisation, as well as helping to overcome the effects of inflammation by suppressing hepcidin.3
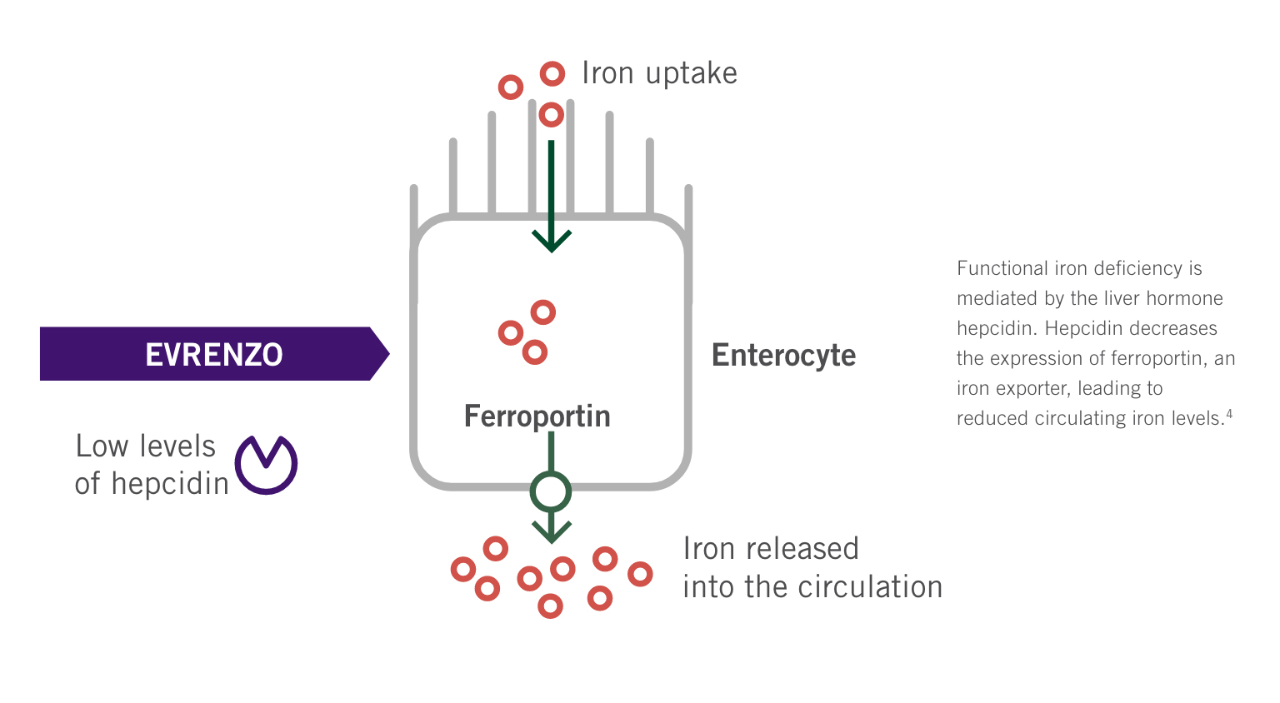
See how EVRENZO works
EVRENZO is indicated for the treatment of adult patients with symptomatic anaemia associated with chronic kidney disease (CKD)3
CKD, chronic kidney disease; EPO, erythropoietin; HIF, hypoxia-inducible factor; HIF-PH, hypoxia-inducible factor prolyl-hydroxylase.
References
By inhibiting HIF-PH, EVRENZO stimulates a coordinated erythropoietic response that:1,2
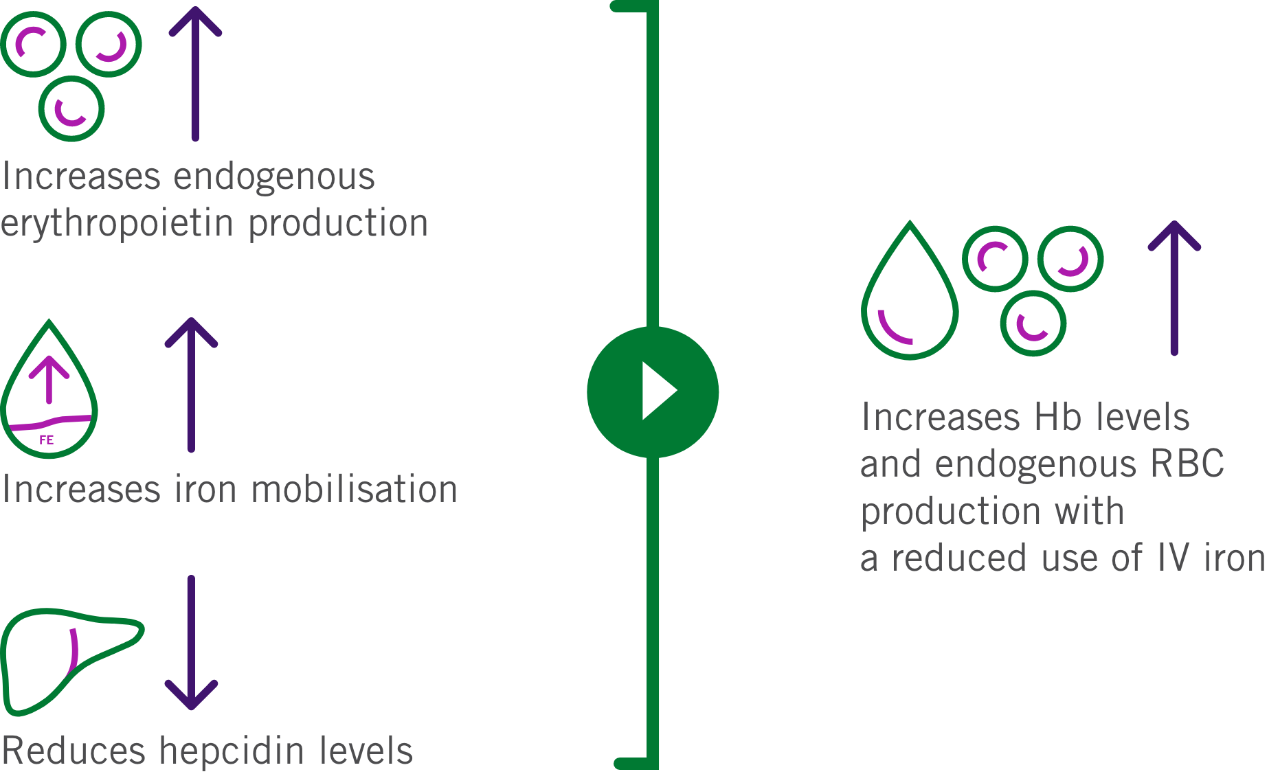
Hb, haemoglobin; HIF-PH, hypoxia-inducible factor prolyl-hydroxylase; IV, intravenous; RBC, red blood cell.
References
Instead of treating the conditon as a deficiency of EPO or iron, EVRENZO addresses multiple factors that contribute to anaemia.1
Targeting the HIF pathway with HIF-PHIs may offer a comprehensive and physiological approach to the management of anaemia of CKD.2
EVRENZO helps patients with anaemia of CKD:
EVRENZO (roxadustat) is indicated for treatment of adult patients with symptomatic anaemia associated with chronic kidney disease (CKD)3
EVRENZO is contraindicated in the following conditions:
-Hypersensitivity to active substance, peanut, soya or any of the excipients
-Third trimester of pregnancy
-Breastfeeding
CKD, chronic kidney disease; EPO, erythropoietin; ESA, erythropoiesis-stimulating agent; HIF, hypoxia-inducible factor; HIF-PHI, hypoxia-inducible factor prolyl-hydroxylase inhibitor; IV, intravenous; TIW, three times weekly.
References
The safety profile of EVRENZO was comparable to that of ESAs,5-9 and AEs were consistent with those commonly seen in patients with anaemia of CKD6-9
EVRENZO offers oral administration with a three times weekly tablet5
AE, adverse event; CI, confidence interval; CKD, chronic kidney disease; Hb, haemoglobin; ESA, erythropoiesis-stimulating agent.
References
EVRENZO™ (roxadustat), 20, 50, 70, 100 och 150 mg filmdragerade tabletter, B03XA05, Rx (särskilt läkemedel), F
▼Detta läkemedel är föremål för utökad övervakning.
Indikationen är behandling av vuxna patienter med symtomatisk anemi associerad med kronisk njursjukdom (CKD). Kontraindikationer är överkänslighet mot den aktiva substansen, jordnötter, soja eller hjälpämne, användning under graviditetens tredje trimester samt amning. Varningar och försiktighet inkluderar risk för allvarliga (inklusive dödliga) kardiovaskulära och trombovaskulära händelser, krampanfall och allvarliga infektioner inklusive sepsis. Ska inte sättas in hos kvinnor som planerar att bli gravida eller under graviditet. Fertila kvinnor måste använda en högeffektiv preventivmetod under behandling och i minst en vecka efter sista dosen. Försiktighet gällande administrering krävs vid måttligt nedsatt leverfunktion och rekommenderas inte vid kraftigt nedsatt leverfunktion. Felanvändning kan leda till en överdrivet förhöjd hematokrit vilket kan leda till livshotande komplikationer i hjärt-kärlsystemet.
Astellas Pharma AB, Box 21046, 200 21 Malmö. Produktresumé 2024-09.
För ytterligare information och förpackningar se www.fass.se.