EVRENZO™ (roxadustat), 20, 50, 70, 100 och 150 mg filmdragerade tabletter, B03XA05, Rx (särskilt läkemedel), F
▼Detta läkemedel är föremål för utökad övervakning.
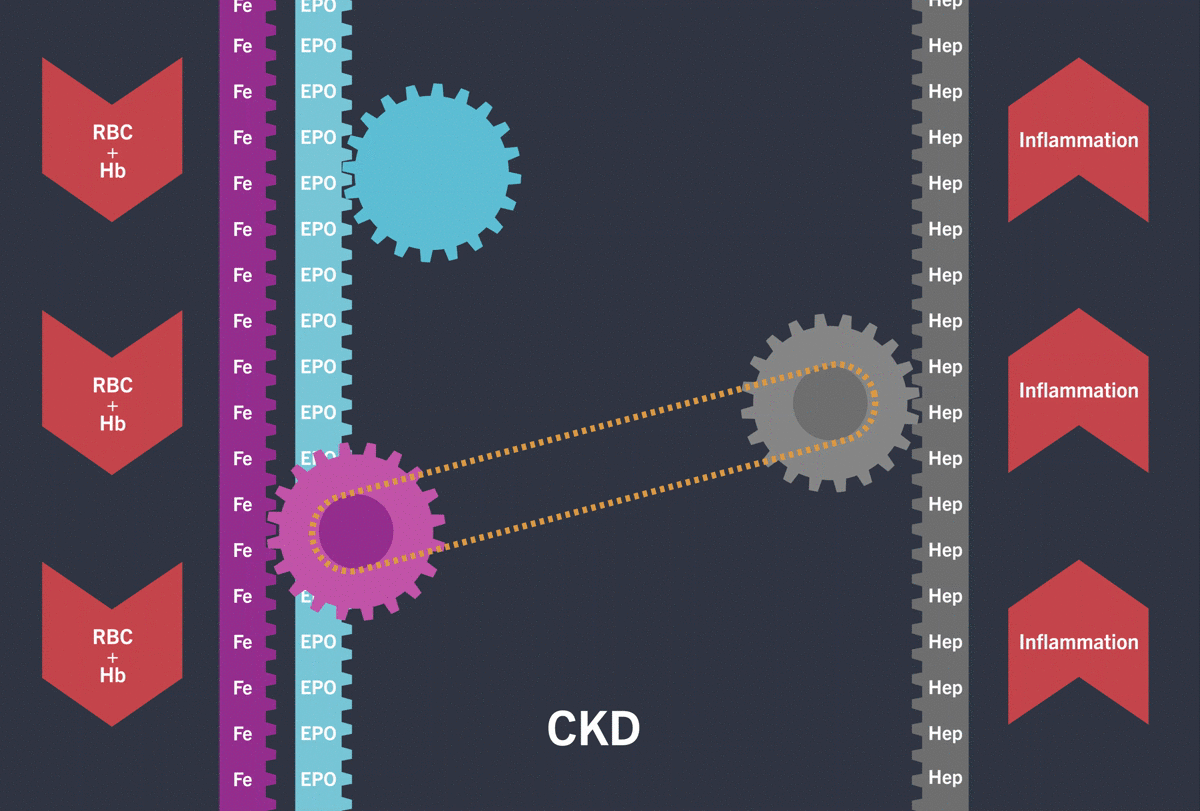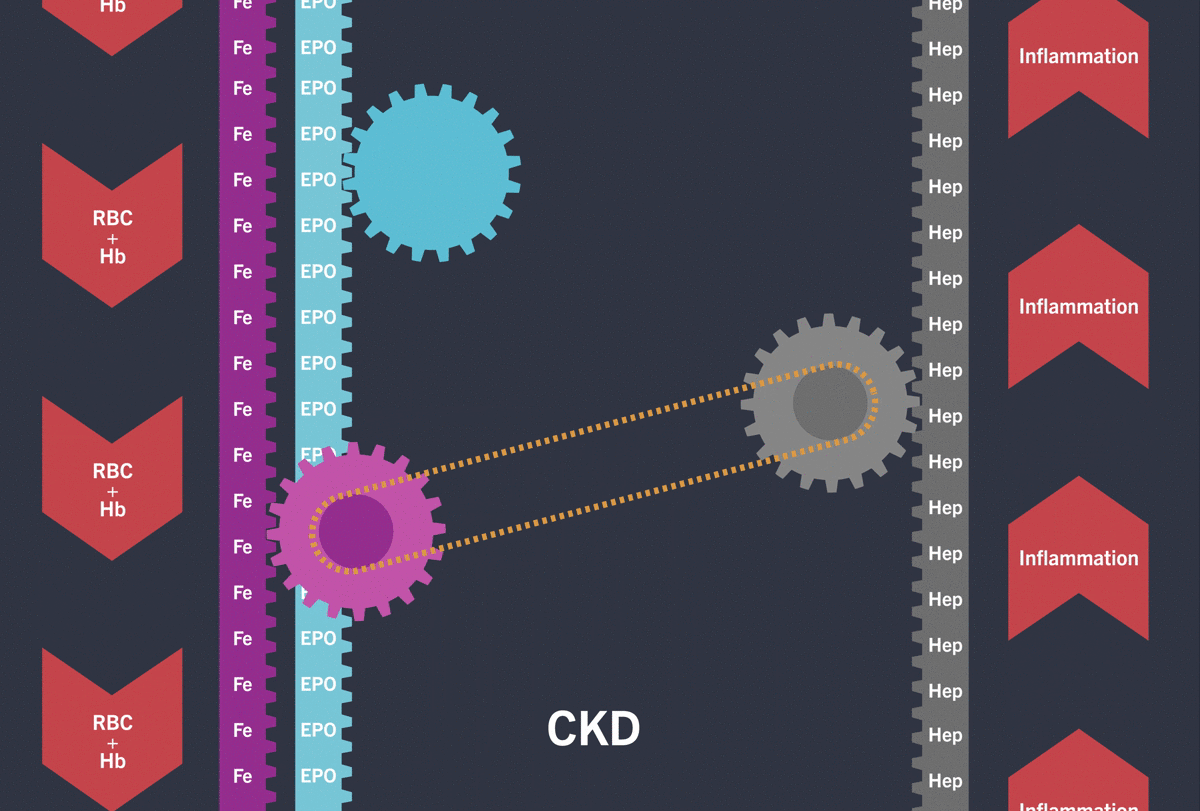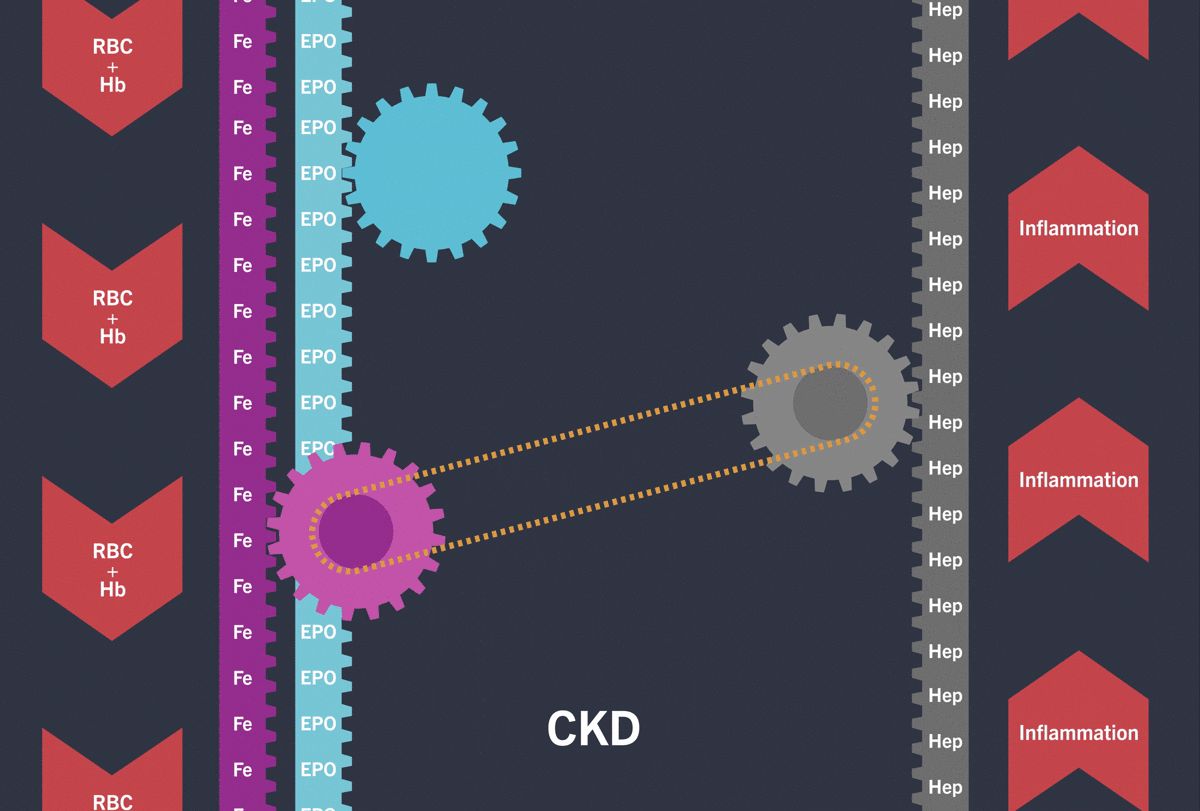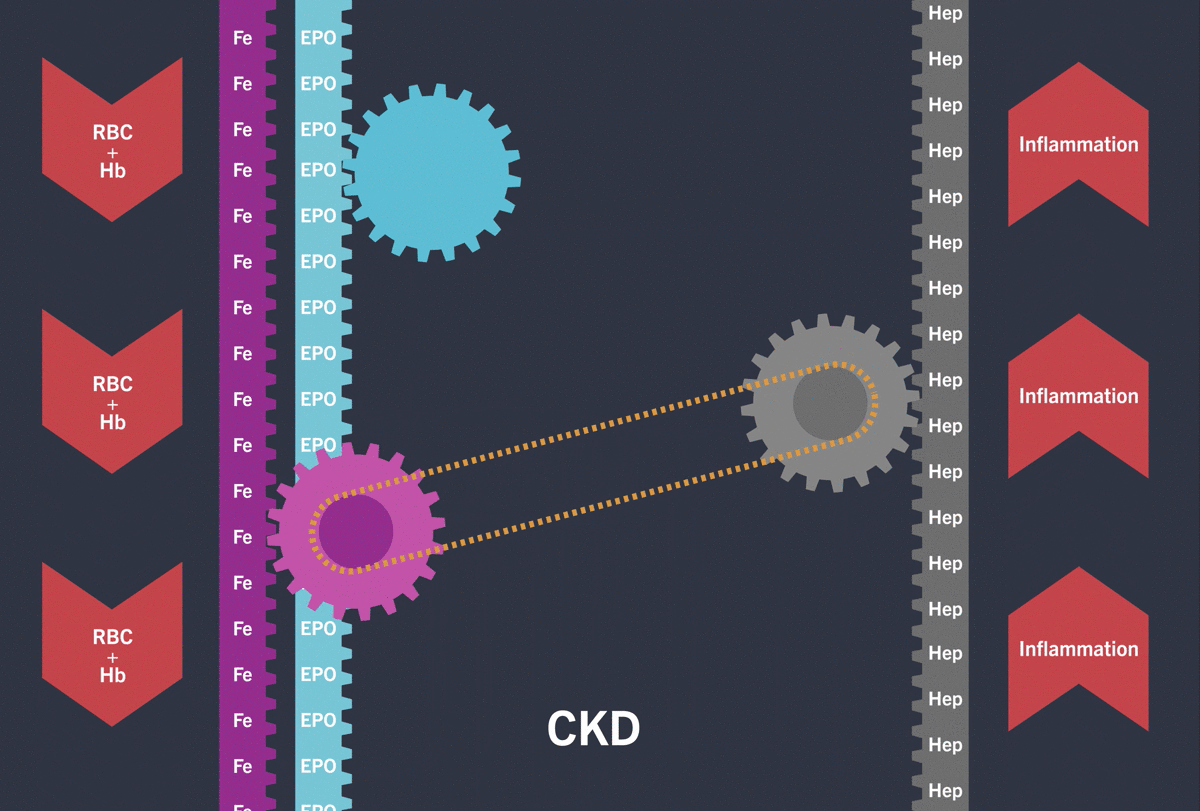
Indikationen är behandling av vuxna patienter med symtomatisk anemi associerad med kronisk njursjukdom (CKD). Kontraindikationer är överkänslighet mot den aktiva substansen, jordnötter, soja eller hjälpämne, användning under graviditetens tredje trimester samt amning. Varningar och försiktighet inkluderar risk för allvarliga (inklusive dödliga) kardiovaskulära och trombovaskulära händelser, krampanfall och allvarliga infektioner inklusive sepsis. Ska inte sättas in hos kvinnor som planerar att bli gravida eller under graviditet. Fertila kvinnor måste använda en högeffektiv preventivmetod under behandling och i minst en vecka efter sista dosen. Försiktighet gällande administrering krävs vid måttligt nedsatt leverfunktion och rekommenderas inte vid kraftigt nedsatt leverfunktion. Felanvändning kan leda till en överdrivet förhöjd hematokrit vilket kan leda till livshotande komplikationer i hjärt-kärlsystemet.
Astellas Pharma AB, Box 21046, 200 21 Malmö. Produktresumé 2024-09.
För ytterligare information och förpackningar se www.fass.se.