IV, intravenous; TIW, three times weekly.
-
EVRENZO offers oral administration with a three times weekly tablet1
Oral administration1
- No need to administer in hospital1
- Reduce the use of IV iron1
- No special storage instructions – can be stored at ambient temperature1
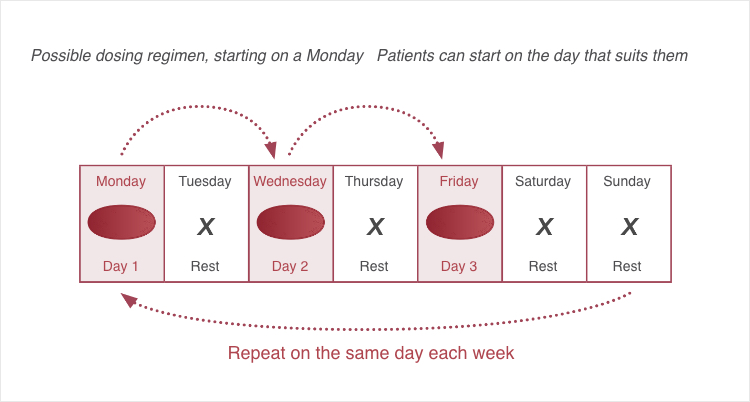
Taken three times weekly not on consecutive days1
Reduced use of IV iron compared to ESA1
EVRENZO treatment reduced the use of iron supplementation compared with treatment with ESA1
References
- EVRENZO SmPC.
-
EVRENZO offers a flexible starting dose regimen
All other causes of anaemia should be evaluated and adequate iron stores should be ensured prior to initating treatment with EVRENZO
ESA-naive patients1
Body weight Recommended
starting dose for
EVRENZO<100 kg 70 mg, 3x per week ≥100 kg 100 mg, 3x per week Patients converting from an ESA1
Conversion of dialysis patients otherwise stable on ESA treatment is only to be considered when there is a valid clinical reason
Conversion of non-dialysis patients otherwise stable on ESA treatment has not been investigated. A decision to treat these patients with EVRENZO should be based on a benefit-risk consideration for the individual patient
- The recommended starting dose of EVRENZO is based on the average prescribed ESA dose in the 4 weeks before conversion
- The first EVRENZO dose should replace the next scheduled dose of the current ESA
- The dose of EVRENZO can be individualised based on the average ESA dose in 4 weeks before conversion
- It is not recommended to combine administration of EVRENZO and ESAs as the combination has not been studied
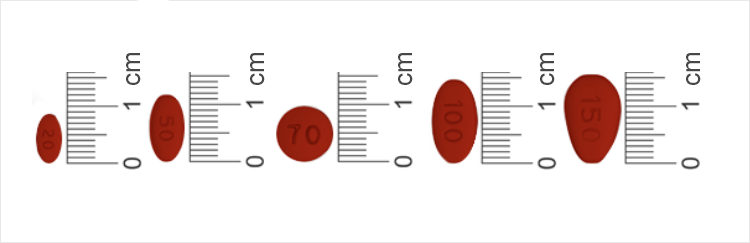
Darbepoetin-alfa IV or SC dose (micrograms/week) Epoetin IV or SC dose (IU/week) Methoxy polyethylene glycol-epoetin beta IV or SC dose (micrograms/monthly) EVRENZO starting dose (milligrams three times per week, not on consecutive days) Less than 25 Less than 5,000 Less than 80 70 25 to less than 40 5,000 up to 8,000 80 up to and including 120 100 40 up to and including 80 More than 8,000 up to and including 16,000 More than 120 up to and including 200 150 More than 80 More than 16,000 More than 200 200 Initial Hb monitoring requirements for EVRENZO are similar to current standard of care for CKD1-3
- Hb levels should be monitored every 2 weeks until the desired Hb level of 10–12 g/dL (100-120 g/L) is achieved and stabilised, and every 4 weeks thereafter, or as clinically indicated1
CKD, chronic kidney disease; ESA, erythropoiesis-stimulating agent; Hb, haemoglobin; IU, international units; IV, intravenous; SC, subcutaneous.
Reference
- EVRENZO SmPC.
- Epoetin alfa Hexal SmPC.
- Aranesp SmPC.
-
Starting patients on EVRENZO
Which patients will you consider first?
Patients not on dialysis?

Patients new to dialysis?

How to start patients on EVRENZO:
In addition to the presence of symptoms of anaemia, other clinical criteria should be evaluated1
All other causes of anaemia should be evaluated and adequate iron stores should ensured prior to initating treatment with EVRENZO1
The appropriate dose of EVRENZO must be taken orally three times per week and not on consecutive days1
The dose should be individualised to achieve and maintain target Hb levels of 10-12 g/dL (100-120 g/L) 1
EVRENZO treatment should not be continued beyond 24 weeks of therapy if a clinically meaningful increase in Hb levels is not achieved. Alternative explanations for an inadequate response should be sought and treated before re-starting EVRENZO1
ESA, erythropoiesis-stimulating agent; Hb, haemoglobin.
Reference
- EVRENZO SmPC.
